Successful products must solve both real business needs and real user problems to deliver value to our clients and their customers.
Before the start of a project, we work closely with clients to fully understand their business needs and goals. With those upfront insights plus our deep understanding of the IVD market, we initiate the process of discovering the needs and goals of users.
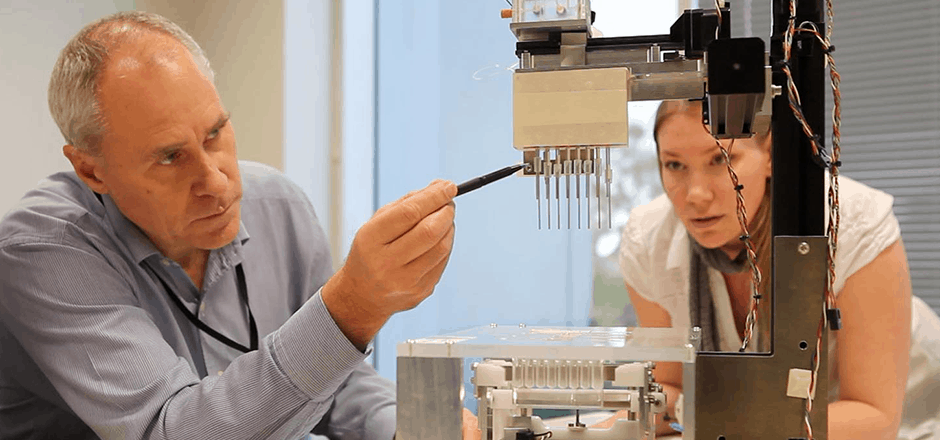
To identify and understand the problems a product must address for the user, our experience design and engineering teams immerse themselves with the people, challenges and user environment to discover the user’s true goals and pain points at the start of the product development process.
This front-end design research involves observing users in context to develop a view of their end-to-end workflow with a product or system at the point of use, including digital, physical and human interactions. These research insights are then analyzed and synthesized into actionable findings and clear objectives that serve as a guide throughout the medical device development process.