In response to the SARS-CoV-2 virus and demand for diagnostic testing, the United States Food and Drug Administration (FDA) called for accelerated development of COVID-19 healthcare solutions and expanded national testing capacity. Under the FDA emergency use authorization (EUA) directive, our client set out to develop a rapid, molecular diagnostic test for the identification of the SARS-CoV-2 virus that was able to be run by non-medical personnel.
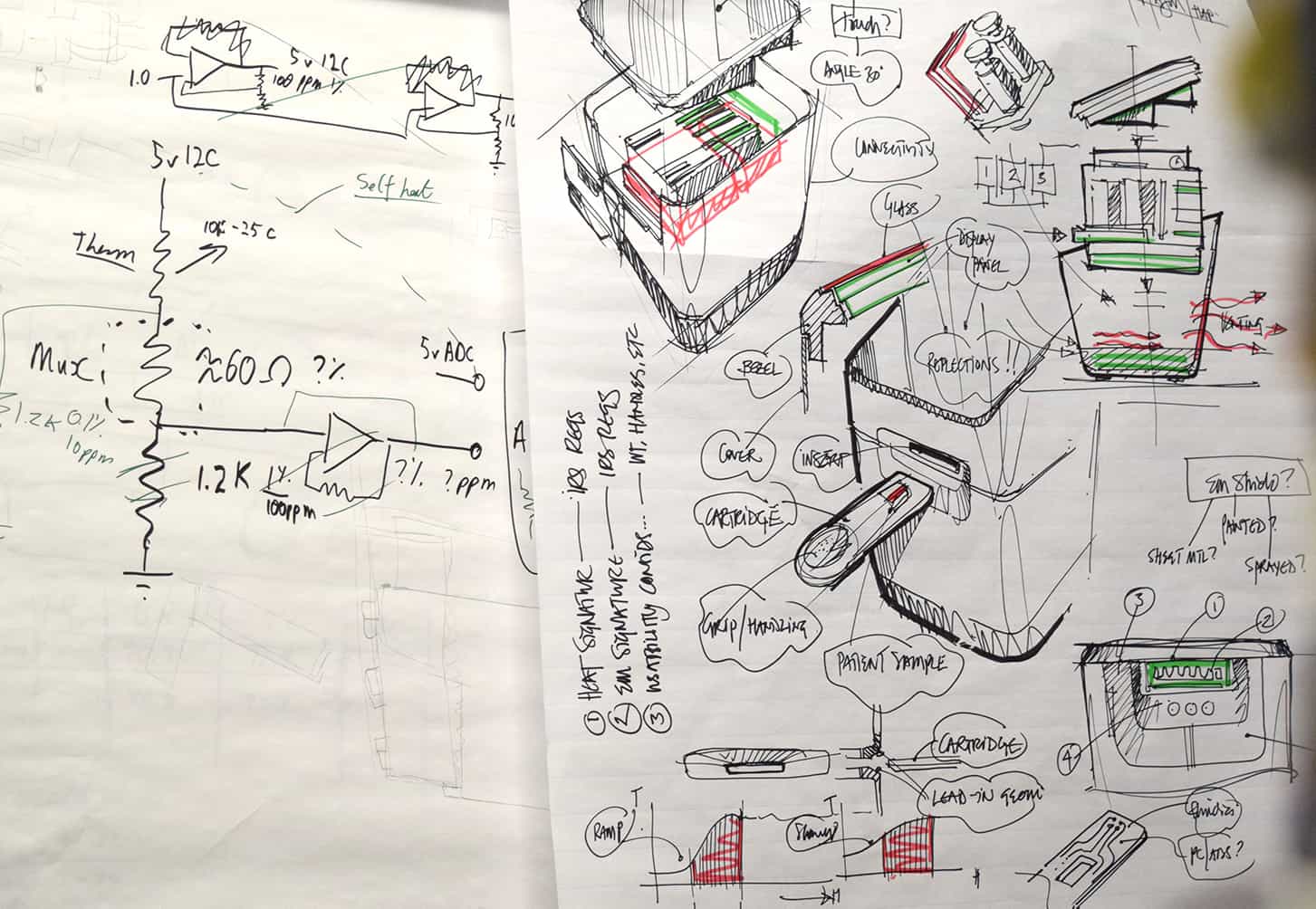
After receiving funding from the National Institutes of Health (NIH) Rapid Acceleration of Diagnostics (RADx) initiative, they engaged Invetech to rapidly convert a manually performed chemistry to an automated consumable-plus-instrument platform that could be used in Clinical Laboratory Improvement Amendments (CLIA)-Waived settings.
The objective was to get to market as rapidly as possible with a fully functioning platform to help address the critical need for diagnostic testing. As part of the product development strategy, design optimization was intentionally deferred to accelerate the delivery on a Minimum Viable Product (MVP).
In under six months, we needed to:
- Establish the platform architecture and define product requirements through transfer to manufacturing
- Address key technical risks associated with integrating an existing mature sample prep protocol and consumable into an integrated POC cartridge
- Arrive at a Minimum Viable Product (MVP) device that met core requirements and was both simple to use and able to scale in production
- Build an instrument that could be scaled effectively to be manufactured in large volumes, shipping at a rate of thousands per month