In a large conference hall at the International Society for Stem Cell Research (ISSCR) conference in Melbourne, a man walked onto a stage in front of 3,000 academic scientists, clinicians and industrial delegates. Addressing the crowd, he introduced himself as Daniel Feller, a father.
Daniel’s son Harry, a cheerful little boy shown wearing big blue spectacles and an even bigger smile, was born with a rare genetic disorder called Usher’s Syndrome. This disorder is characterised by the loss of hearing from birth, and the progressive degeneration of his retina which will lead to blindness at adolescence. While cochlear implants have given Harry the ability to hear, there is currently no cure for the impending loss of his eyesight.
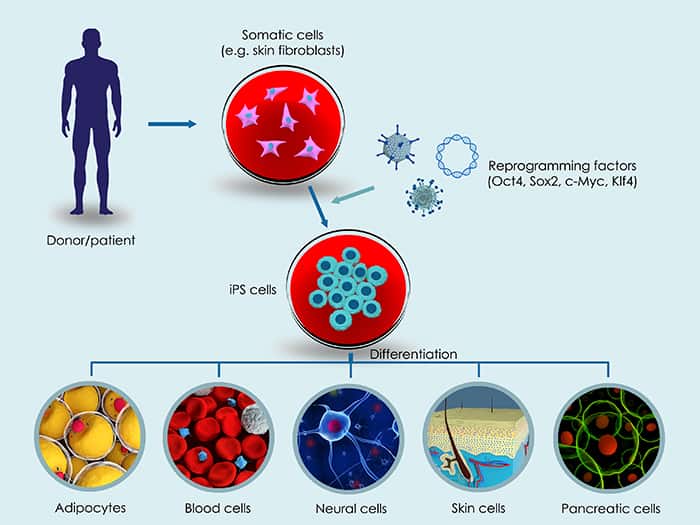
Doing what any loving parent would do, Daniel and his wife sought treatment for their son, now 7 years old. The couple reached out to researchers at the Centre for Eye Research Australia (CERA), who were working on developing a cutting-edge stem cell treatment for inherited eye diseases, using induced-pluripotent stem cells (iPSCs) derived from the patient.