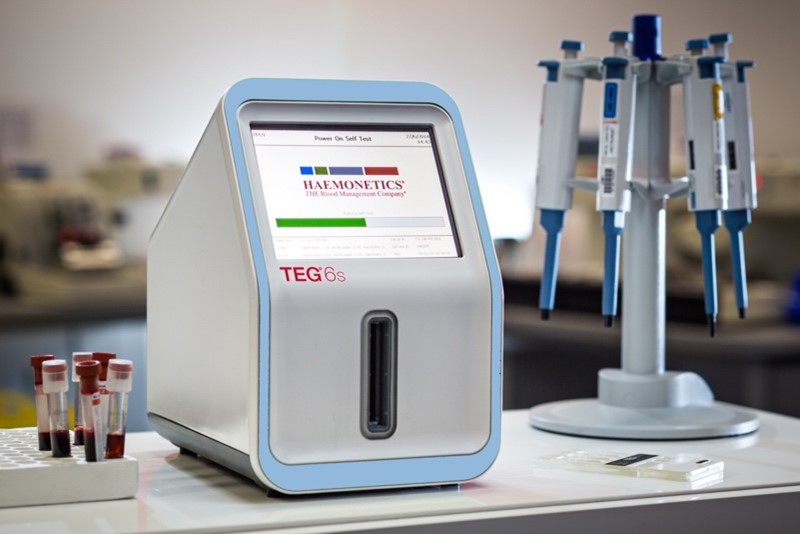
Haemonetics®: TEG® 6s Hemostasis Analyzer System
This next generation instrument brought the client’s breakthrough technology from the clinical lab to the point of care for life-saving results.REGULATORY
REQUIREMENTS
IVD directive, CE, EN61010, UL61010A, FCC, EN61326, ROHS
EXPERTISE
LEVERAGED
Fluidics, Optics, Motion, Consumable Design, Usability/HFE, Connectivity & Systems Integration, Contract Manufacturing
Bringing Breakthrough Technology from the Lab to the Point of Care
Having real-time awareness of a patient’s blood clotting ability, or hemostasis, in an emergency or surgical setting is crucial for doctors to be able to maintain patient coagulation equilibrium. Oftentimes, hemostasis analysis is completed in a hospital’s central laboratory, separate from the surgical theater. This arrangement wastes valuable time by requiring operating room staff to leave the surgical theater to access these critical test results.
Haemonetics, a healthcare company dedicated to providing innovative blood management solutions, partnered with Invetech to design and manufacture the TEG® 6s Hemostasis Analyzer System. This system is a compact diagnostic instrument that provides clinicians with rapid, comprehensive and accurate identification of a patient’s hemostasis condition in a surgical theater, laboratory
or point of care setting.
The TEG® 6s Analyzer was introduced to the market in 2014 and has been in full production by our contract manufacturing team since its launch. The instrument went on to win the Good Design Award in 2016 for its compact, minimalist design with all-in-one cartridges, resonance and sensing algorithm, large touchscreen, and user-friendly interface.
Featuring an innovative, all-in-one cartridge, the TEG® 6s system delivers the same quality test results without the complicated test preparation process. The instrument allows clinicians to drive personalized, clinically and economically sound treatment while also allowing them to make decisions with confidence.
Integrating New Optics Technology Application
For the first time in a hemostasis analyzer, the TEG® 6s Analyzer introduced a resonant frequency technology with optics analysis. The blood sample is suspended and its coagulation is measured by its response to vibrations over a wide spectrum of frequencies.
This response is then analyzed using a series of algorithms and appears to the end user as an easy-to-read result on the instrument’s touchscreen display – a design upgrade from the external data processing required by the previous version. Having the algorithms function
correctly given the processing constraints of the embedded system within the instrument presented various challenges.
To optimize the development process, we created a framework on a PC that exactly replicated how the algorithms are calculated by the instrument’s firmware. In doing this, we could tune and debug the system by reprocessing test files captured on both the instrument as well as synthesized files for rarer, out-of-range cases. During this process, we were also able to make improvements such as speeding up the key matrix operations by two orders of magnitude.
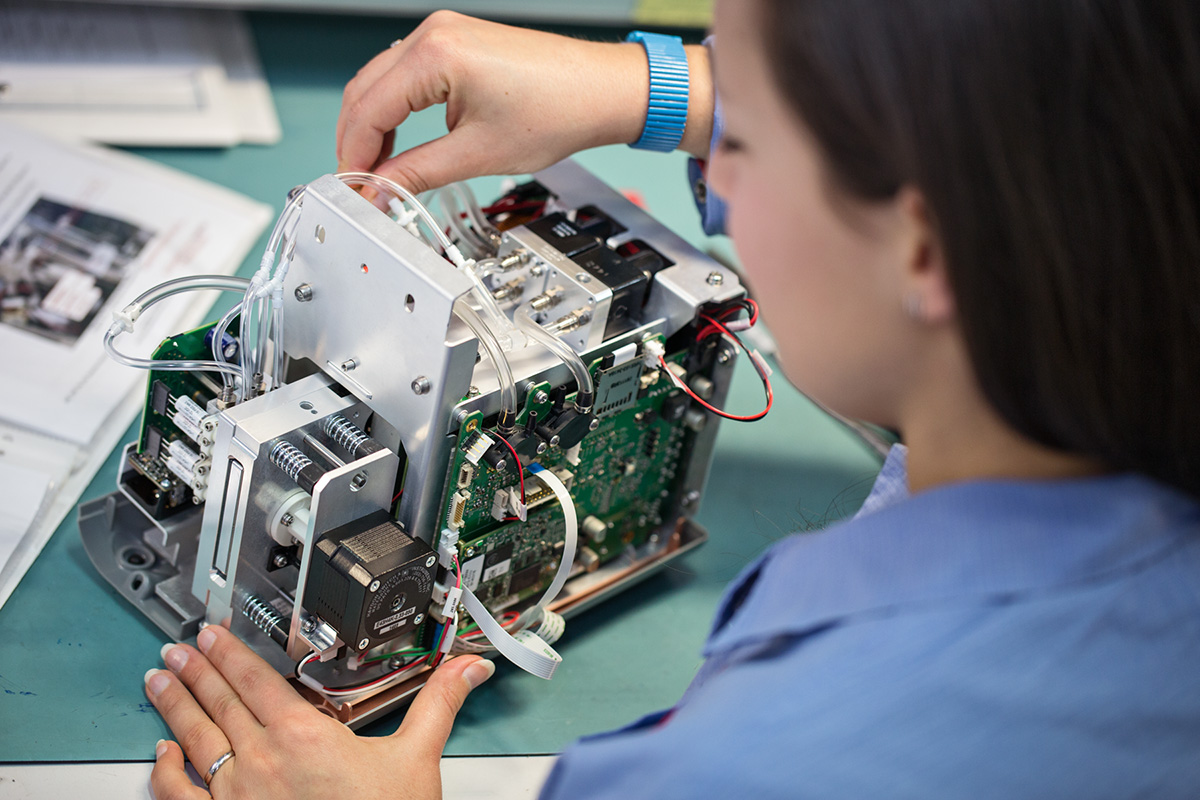
Creating Better Solutions with User Insights
Throughout the instrument’s development, prototype units were deployed into local hospitals to gather user feedback that we translated into appropriate design modifications. Examples of these modifications include rearranging how data is displayed on the touchscreen, color coding test results and adding a timer to the screen while tests are being conducted.
We also worked closely with
Haemonetics’ service team, who interact directly with the Haemonetics’ end users and know the everyday issues that arise after an instrument has been released.
The flow of information went both ways: while we listened to the service team’s observations on potential issues their customers might have, they became familiar with the TEG® 6s Analyzer so that they could better service it once it was launched.

Moving from Concept Development to Contract Manufacturing
Our contract manufacturing team worked directly with our designers and engineers early on to ensure the final product design was easy to assemble and eliminated risks associated with the assembly and manufacturing processes.
This included:
- Implementing a preliminary production process and completing a PFMEA (Process Failure Mode Effects Analysis) during the design development stage. Refining the product’s design early to account for any design-related assembly issues helped to avoid time-consuming changes to the manufacturing processes later on.
- Securing inventory and establishing
- the production line while the design was still developing. This enabled the team to eliminate several issues that would have impacted product quality, delivery and ramp up.
- Building prototypes for verification testing and clinical trials prior to commercial launch.
- Leveraging tools from the Fortive Business System (FBS) to decrease manufacturing time from two days to one day, while maintaining the high level of quality required for a Point of Care platform.
Through this collaboration, we were able to address potential manufacturing issues upfront so we could quickly transfer the product into production and meet the client’s desired launch date
Ongoing Support to Ensure Seamless Production
The TEG® 6s Analyzer has been in full commercial production by our contract manufacturing team since its introduction to the market in 2014. We continue to provide the following ongoing support to ensure seamless production:
- Proactively addressing supply continuity risks by focusing on early identification of obsolescence and verifying replacement parts.
- Continuously monitoring the instrument’s field performance to inform design and process improvements.
- Managing design and supply chain changes to reduce COGS, including low-cost region
- (LCR) sourcing where appropriate.
- Closely collaborating with the client’s stakeholders across the globe, including their engineering, service, quality, regulatory, supply chain and marketing teams.
To adapt to varying demand and forecast changes, we also provide flexible production processes. This includes supporting rapid, unexpected fluctuations in demand – as certainly was the case with the COVID pandemic – by working through supply chain and production processes to scale up and revise the production line as needed.
More Case Studies
Below are a selection of case studies highlighting the range of problems we’ve solved to deliver innovation and business value for our clients.

Life Sciences & Biotech
Not all innovative or complex technology is used in the clinic, but these systems benefit from many of the same principles used.
Mid to High Throughput Instruments
Whether we support the entire product or take lead on a subsystem, full instrument architecture is critical to success.
Benchtop & Distributed
Translating technical workflows of an assay into an automated process performed within a device.

Custom Process Automation
Transition from clinical to commercial scale manufacturing with our production-ready manufacturing and automation solutions.