Closed Manufacturing System for Autologous Cell Therapy Production
Breakthrough manufacturing automation for cell expansion, formulation and working cell bank dispensing paves the way for treatment of debilitating illness.Client: Confidential
Product: Manufacturing Automation Solutions for Autologous Cell Therapy Production
REGULATORY
REQUIREMENTS
OSHA, IEC/UL 61010-1, FCC – CFR 47 FCC 15 sub. B., Manufacturing Execution System (MES) certified and 21 CFR Part 11 compliant
EXPERTISE
LEVERAGED
Laboratory Services, Industrial Automation, Design for High-Throughput Manufacturing, Medical-Grade Plastic Disposable Design, Fluidics, Design for Serviceability, Usability/HFE
Planning for commercial scale-out of an autologous cell therapy
A common challenge in the emerging cell and gene therapy industry is translating manual production methods to clinical and commercial-scale manufacturing. Recently, a clinical-stage biopharmaceutical company was looking to commercialize an autologous Dendritic cell therapy, an immunotherapeutic approach for treating and preventing cancers and autoimmune diseases. The company approached Invetech during Phase II clinical trials to commence development of scaled-out GMP manufacturing solutions.
In briefing Invetech, two key challenges were identified:
- The therapy needed to be produced consistently and repeatedly at a lower cost
- The manual benchtop process was required to be closed in a way that was suited to automation and scale-out yet supported effective biochemistry.
Invetech was engaged to develop a complete manufacturing system that would become the backbone of the client’s entire purpose-built manufacturing facility plans.
Single-Use Set (SUS) development
Successfully closing the manufacturing process is key in scaling therapies. For the therapy being scaled, this was achieved through the development of closed SUSs. Seven separate process stations, each with a dedicated SUS with sterile assemblies of OEM and custom plastic components, were designed and implemented.
The SUSs were designed to maintain cell viability while performing many functions, including:
- Structural temperature control
- Fluid control
- Internal humidity
- Vapor management
- Centrifugal and magnetic separation
- Electric field management
Successfully closing end-to-end manufacturing allowed production in lower-class cleanrooms, and multiple patient processes to be run in parallel in the same facility resulting in a substantial decrease in the cost of therapy.

Innovating the cell therapy market
During this project, Invetech developed methods to undertake all major cell therapy processing steps within closed SUSs, as well as some process steps specific to the client such as RNA extraction from tumor homogenate and amplification. Through the collaboration with Invetech, the client pioneered taking SUSs with closed processing to the U.S. Food and Drug Administration (FDA).
In discussions with FDA, there was support for furthering the development of this equipment and associated consumables to enable processing of multiple patient samples in closed SUSs in the same manufacturing area. The innovative step of utilizing assets to enable high-value processing on expensive equipment and low-value processing in simple incubators is now a recognized industry strategy.

Collaboration is key on complex projects
Invetech collaborated closely with the client to ensure development decisions were considered from both business and technical perspectives. The teams had regular steering committee meetings and weekly teleconferences.
The client’s team visited Invetech’s offices to test and assess prototypes, after which prototype systems were shipped to the client for ongoing in-house process development. Invetech also installed one of their own engineers in the client team for nine months.
Managing risk with relationship and modules
Throughout the development, Invetech managed risk using a number of tools and methodologies including the consistent use of risk watch lists, failure modes and effects analysis (FMEA) and sophisticated project tracking and analysis tools. Utilizing FMEA enabled the Invetech team to understand and characterize risks early. Risks were then addressed through multiple agile prototype and test rounds, and through
seeking external advice, establishing partnerships and working with the client and others to explore best approaches. Invetech also implemented the use of common modules in this project by identifying core elements of functionality – such as mechanical hardware, control systems and consumables – that were utilized across several of the seven platforms.
Designing for error reduction
In developing a complete manufacturing system, Invetech’s solutions extended to the larger environment beyond individual machine automation, including minimizing equipment set-up and tear-down times, developing intuitive and simple-to-use touch-screen interfaces and designing dedicated support equipment such as trolleys for handling the heavy components.
Full-scale prototypes of each system were developed with every interaction tested, assessed and optimized through the entire manufacturing chain.
“The automation developed for our immunotherapy lowers labor and production costs. With reduced risk of process inconsistency and contamination due to the use of robotics in a closed, airtight system, automation of the therapy addressed the manufacturing challenges of personalized immunotherapies, and could potentially meet the needs of large patient populations in cancer and infectious diseases.”
– President and Chief Executive Officer, Invetech Biopharmaceutical Client
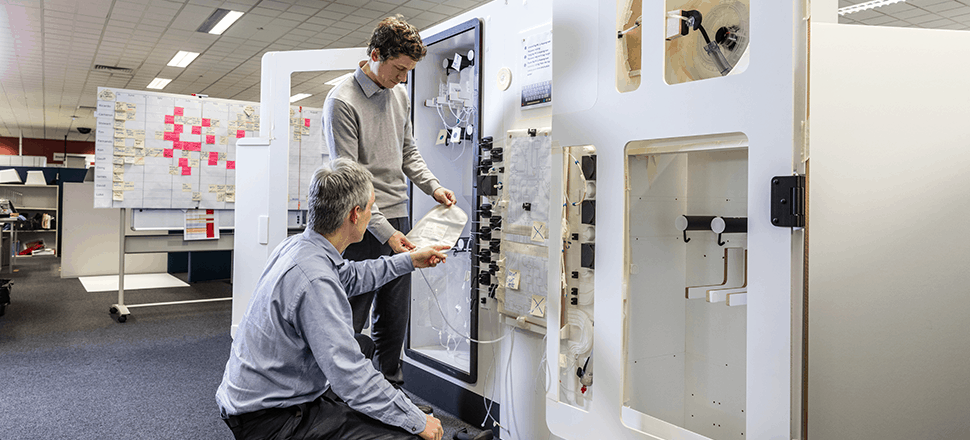
The role of connectivity & system integration
The platforms were designed to connect to a manufacturing execution system (MES), which allows for system connectivity and control through the entire facility. The platforms were designed for secure remote monitoring and control, including software update capability. This allows a single control center to manage the configuration of every platform globally and for functionality like remote fault assessment.
Invetech’s role
The value Invetech delivered was comprehensive, including technology innovation, novel automation solutions, and substantial IP in the cell therapy manufacturing automation domain.
The therapy has not yet been introduced to the market, but the technology developed will be adapted by the client for developing other therapies. Invetech’s approach to overcoming the client’s manufacturing challenges will have an ongoing impact on the cell therapy manufacturing systems of the future.

More Case Studies
Below are a selection of case studies highlighting the range of problems we’ve solved to deliver innovation and business value for our clients.

Life Sciences & Biotech
Not all innovative or complex technology is used in the clinic, but these systems benefit from many of the same principles used.
Mid to High Throughput Instruments
Whether we support the entire product or take lead on a subsystem, full instrument architecture is critical to success.
Benchtop & Distributed
Translating technical workflows of an assay into an automated process performed within a device.

Custom Process Automation
Transition from clinical to commercial scale manufacturing with our production-ready manufacturing and automation solutions.