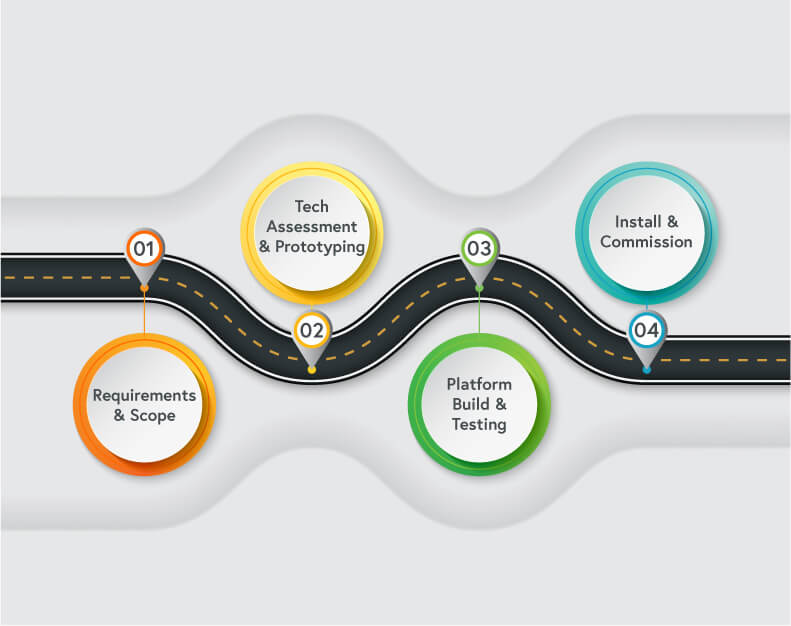
What is a manufacturing roadmap?
A good cell and gene therapy (CGT) manufacturing roadmap provides flexible guidelines to advance automation. It’s a living document and will be refined many times as cell therapy manufacturing needs and processes evolve.
The roadmap should first be implemented during the preclinical phase to encompass the complete journey. The destination is full commercialization, and each manufacturer must understand how to get there.
CGT manufacturers must review the technology landscape to understand their choices. For example, they may need an instrument that does not actually exist and will have to decide whether it is more cost-effective to build one from scratch or find another option.

The key is mapping out the entire manufacturing process and developing a clear understanding of what needs to be done and when.

