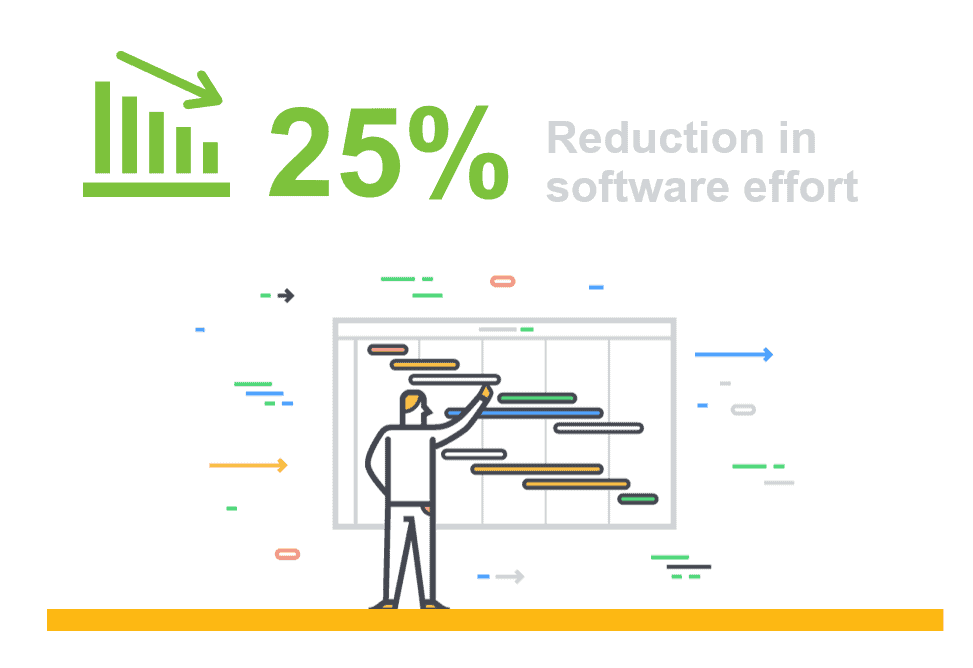
Digital health continues to be a work in progress, but there have been some great developments in recent years. For example, the introduction of electronic health records (EHR) has greatly improved the collection, access and analysis of medical data for both physicians and patients. Cloud-based information systems have been a major contributor in driving this EHR adoption in both large and small institutions. However, there remain many areas of healthcare data handling and record keeping where system improvements are desperately needed.
The COVID-19 pandemic has highlighted the current inefficiencies in easily managing data exchange and analysis. With the associated rapid growth of decentralized testing locations, the ability for quick data access and analysis is more important than ever before.