Development Strategy for Diagnostic Devices
Product Development Strategy
Successful products must solve both real business needs and real user problems to deliver value. Browse the articles, white papers and videos below for insights and resources on designing for the user, implementing an agile development approach, applications for machine learning and more.

ARTICLE
Diagnostic Product Development in a Post-Pandemic Era – New and Emerging Trends
Post COVID-19, accelerated development timelines, flexible manufacturing and the elevated role of the supply chain will be product development best practices.

WHITE PAPER
Guide to Experience Design for IVD Product Development
Incorporating experience design into the product development process results in more engaged users.

ARTICLE
Designing for an Increasingly Demand-Driven Healthcare Environment
The rate of change within the healthcare and diagnostics landscapes are unprecedented, presenting both challenges and opportunities for IVD manufacturers.
ARTICLE
Are Lab-Equivalent Results Required for Point of Care Testing?
Best practices for evaluating the design trade-offs that may need to be made to achieve acceptable levels of accuracy, sensitivity, cost, and time to result and best match the needs for a particular test.

WHITE PAPER
User-Focused Product Definition for IVD Market Success
Designing for the user is becoming increasingly necessary for in-vitro diagnostic (IVD) manufacturers. Successful products must solve real user problems to deliver value, so discovering the user’s true goals and needs at the start of the product development process is essential.
ARTICLE
Machine Learning Applications in Medical Devices
Despite current constraints, the clear productivity gains available from well-designed and targeted machine learning systems will drive innovation in medical device design.
Need a product development partner?
Your core technology is powerful – and even more so once fully engineered and commercially-ready. We can help.
GUIDE
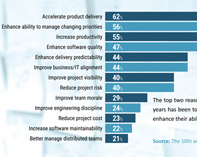
What is Agile for IVD Product Development?
The IVD industry is increasingly challenged to deliver new instruments to market while dealing with slipping timelines, shrinking budgets and resource constraints. Adopting agile methodologies is one way instrument developers can overcome some of these challenges.

VIDEO
Why IVD Product Development Needs to Become Agile
The IVD industry is increasingly challenged to deliver new instruments to market while dealing with project delays, shrinking budgets, and resource constraints.

ARTICLE
The Role of Experience Design in POC Product Development
A recap of the presentation Cece O’Connor, Invetech’s Global Director of UX Strategy & Design, gave at the 24th International Molecular Medicine Tri-Conference.

ARTICLE
Delivering Innovation Through Human-Centered Design
While innovation is a top priority for healthcare companies and IVD manufacturers, it continues to be a frustration for organizations in its execution.

VIDEO
How is Experience Design Influencing IVD Innovation?
Invetech’s annual diagnostics breakfast event during the 2017 AACC Annual Meeting & Expo featured a panel discussion among IVD industry leaders from Bio-Rad Laboratories, Hologic and MeMed Diagnostics exploring the topic of designing diagnostics for the end user.

ARTICLE
How Immersive Reality is Improving Healthcare Product Development
The ability of Immersive Reality to communicate complex ideas and concepts to a broad audience offers benefits as part of the design and development process.
Schedule a conversation
We want to help turn your vision into reality. Contact us today to get started.