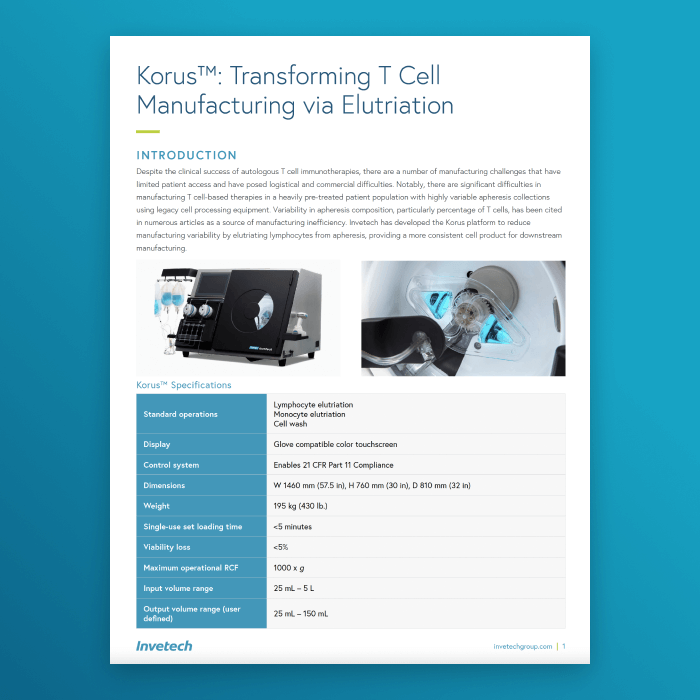
Korus™
Elutriation. Washing. One system.

Resources
Korus™ Application Note: Transforming Monocyte Based Cell Therapy Manufacturing via Elutriation
Invetech presents Korus™ data for the efficient and effective enrichment and concentration of a monocyte cell population, starting with a healthy donor leukopak.
Transforming T Cell Manufacturing via Elutriation with Korus™ >
In this on-demand webinar, industry experts share challenges encountered when closing and automating ancillary processes, and how improved scalability and reproducibility were achieved by moving to closed automated platforms.
Whitepaper: The effects of elutriation versus wash >
Our data set shows that samples prepared with the Korus™ elutriation protocol result in significantly higher T cell selection recovery and growth in comparison to samples prepared with the standard CS5+ wash protocol.
Video: Introducing the Korus™ >
The Korus™ system is designed to get your manufacturing process off to the right start by providing you with a cleaner cell population.
Brochure: Manufacturing Solutions for Your Cell and Gene Therapy Journey >
Invetech offers a wide variety of custom solutions and ready-to-run systems for cell and gene therapy. This brochure gives a succinct overview of what you can expect from Invetech.
Datasheet: Korus™ Counterflow Centrifugation System >
The Korus™ datasheet gives an overview of the Korus™ system. It details the hardware, software, and single-use set specifications.
FAQs
How does Korus™ separate cells?
The Korus™ uses the process of elutriation to separate cells. Learn more about the elutriation process.
Do you have data evaluation red blood cell (RBC) contamination in the Korus™ system?
Korus data captures RBC and platelet depletion.
What is the cell capacity of Korus™?
It depends upon cell type, but as an example each 50 mL chamber can accommodate roughly 15 billion cells per chamber depending on cell type. With 2 chambers, a total of 30 billion cells.
Can I specify the output volume?
Yes, between 25 mL to 150 mL, independent of the input and wash volumes.
How low have you pushed the output volume range?
25 mL is the specified output lower limit, but in certain circumstances it is possible to go as low as 15 mL. For further information on how the Korus™ can be optimized to fit your protocol, please contact us here.
What’s the typical recovery?
Our complete dataset including typical recovery can be viewed here.
How long do these runs take?
For most applications, the process time will be under 60 minutes.
Are there specific buffers that should be used with the Korus™?
We are accumulating data using standard buffers such as HBSS. Invetech can assist with buffer and parameter selection. For more information, please contact us here.
Can I optimize Korus™ to balance my purity and recovery requirements?
Yes, the Korus™ software enables optimization of your process parameters such as centrifuge speed, counterflow rate, counterflow ramp rate and elutriation volume so that you can balance your purity and recovery requirements.
Is the Korus™ a fully closed system? How do I attach my cells to the Korus system?
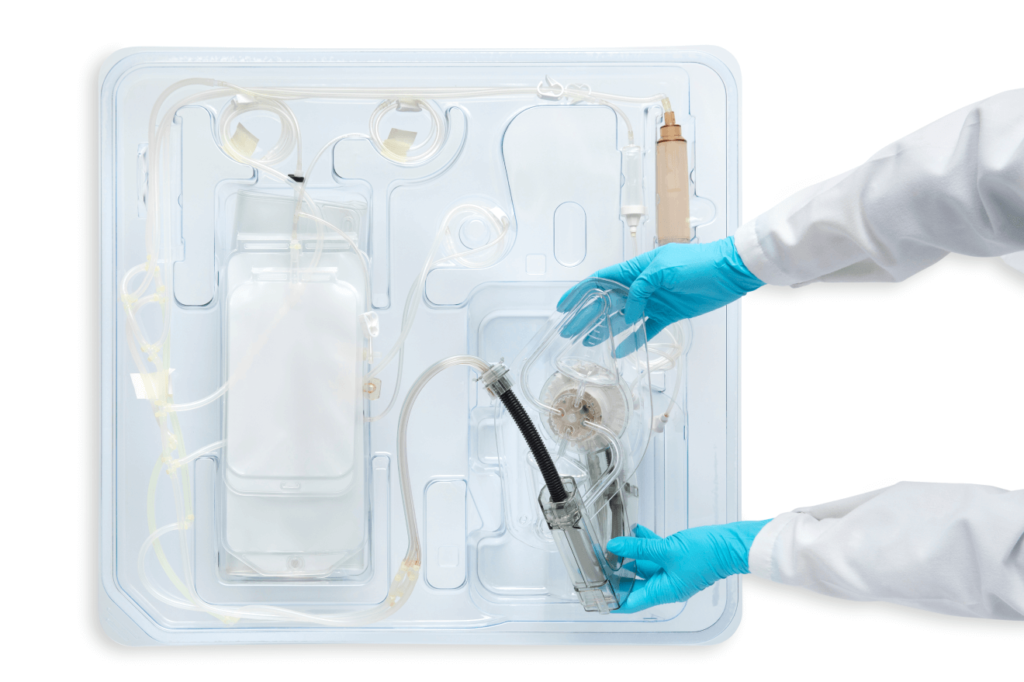
The disposable Korus™ single use set is closed, sterile and is composed of weldable tubing.
How difficult is it to put the single-use set on?
The single-use set takes fewer than five minutes for you to install. It comes neatly packaged in a tub, with aseptic connections already intact, and is designed to be intuitively connected to the Korus™.
What is the process for replacing Elutra?
Korus™ can attain the same goals as Elutra and additionally perform cell wash and concentrate. The parameters however will likely be different so expect some fine tuning. Contact us to see how Korus can address your elutriation needs.
How much does it cost?
For pricing and other sales related questions, please contact us.
The Korus system by Invetech is for research, laboratory or cell therapy manufacturing processes use only. It is not intended as a medical device in therapeutic or diagnostic procedures. Customers are responsible for validating the use of Korus technology within their process or therapy. Consult all instructions, terms and warnings prior to use of the solutions and products. Failure to do so can result in damage or serious injury, including death. Ready-to-run turnkey systems may require minor configuration before sale and use depending on customer needs.