Korus™
Elutriation. Washing. One system.

Prepare cleaner cell populations
with the Korus™ system
Monocyte elutriation
Up to 92% monocyte recovery and 72% monocyte purity. Isolation of monocytes
for downstream processes such as dendritic cell (DC) maturation.
Lymphocytes
Monocytes
RBCs
Platelets
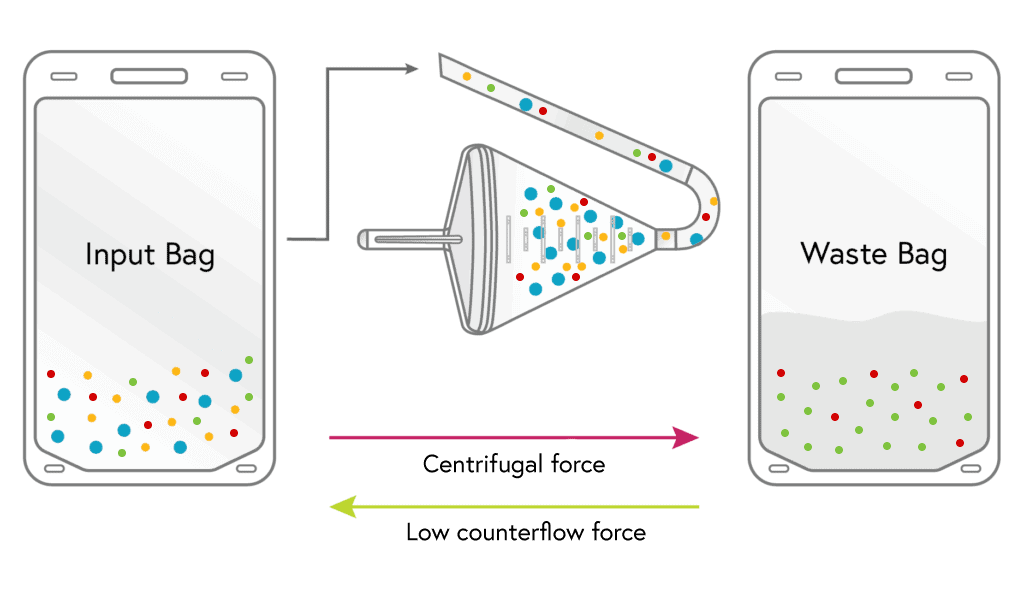
1
Loading
Cells from the input bag enter the chamber and are held there as excess fluid along with most of the platelets and a minor portion of RBCs flow to waste.
2
ELUTRIATION
Media flows into the chamber which causes the lymphocytes and many of the RBCs to leave the chamber, these are sent to either the transfer fraction bag or waste.
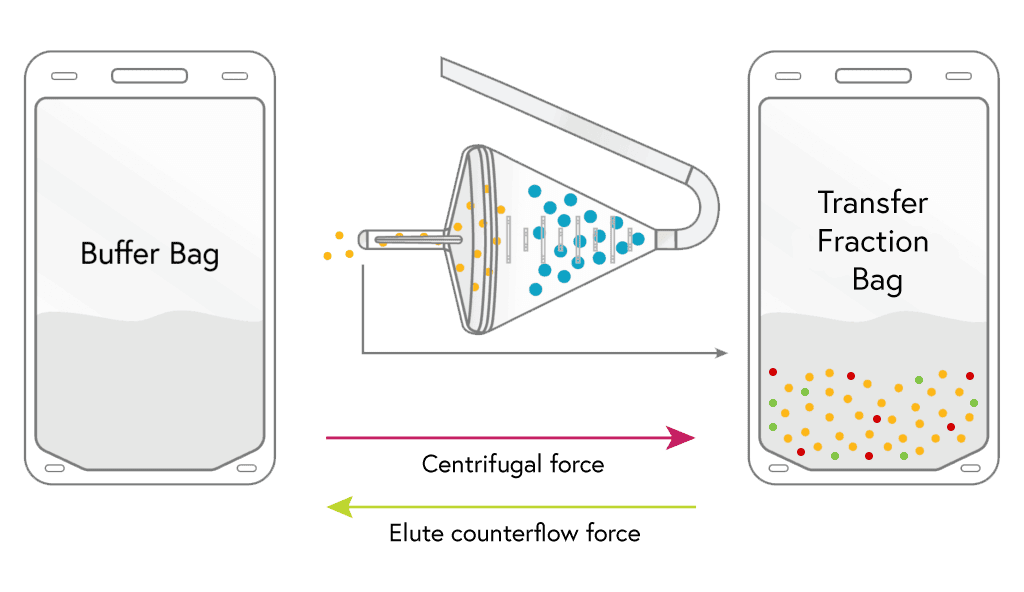
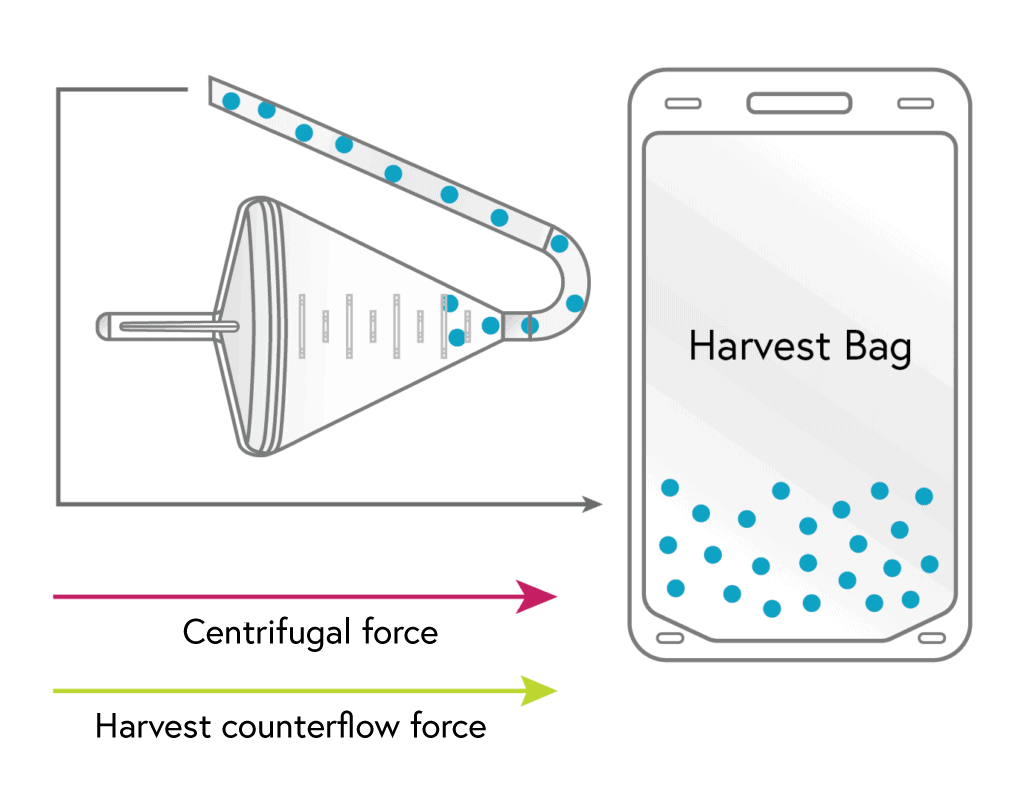
3
HARVEST
With most of the other cell types removed, the remaining monocytes are flushed from the chamber to the harvest bag.
*Standard media exchange capability: Korus™ streamlines the manufacturing workflow with the media exchange as well as volume reduction as a standard feature on Korus. Korus can help you achieve the desired cell concentration, volume, and suspension media through a fully automated process.
Lymphocyte elutriation
Up to 85.2% lymphocyte recovery and 90.3% purity, providing a cleaner starting material for CAR-T or TIL manufacturing. Can also be used for PBMC enrichment.
Lymphocytes
Monocytes
RBCs
Platelets
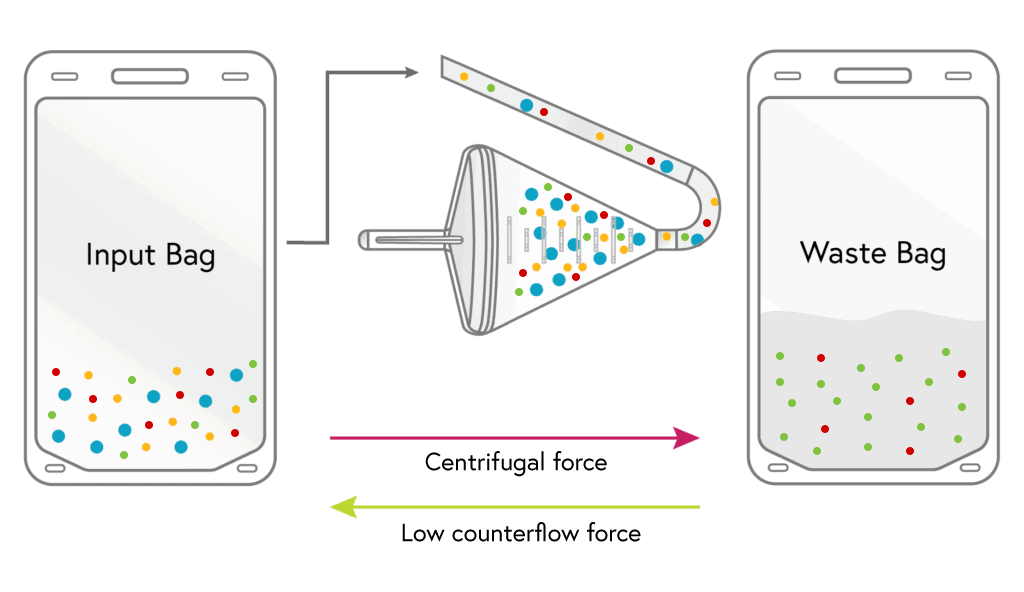
1
LOADING
Cells from the input bag enter the chamber and are held there as excess fluid along with most of the platelets and a minor portion of RBCs flow to waste.
2
ELUTRIATION
Media flows into the chamber which causes the lymphocytes and many of the RBCs to leave the chamber, these are collected in the ‘fraction bag’.
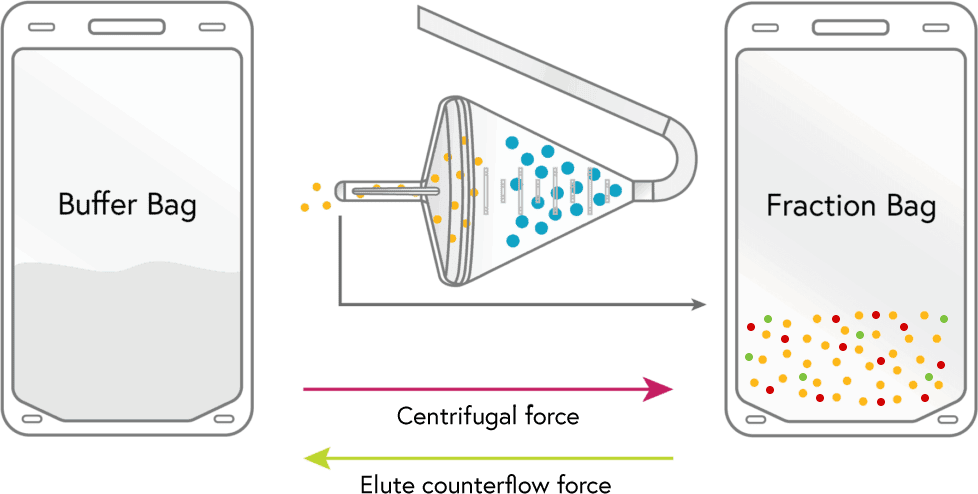
3
CONCENTRATION
Lymphocytes from the fraction bag are loaded into the chamber where they are held and concentrated down much like a filter. During this step the remaining platelets and up to 50% of the RBCs are removed.
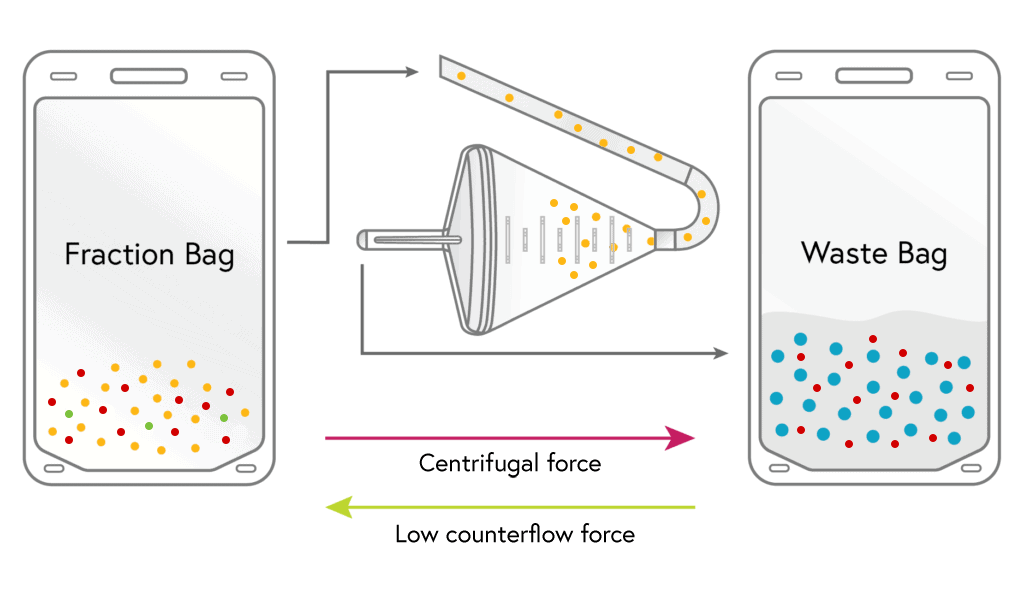
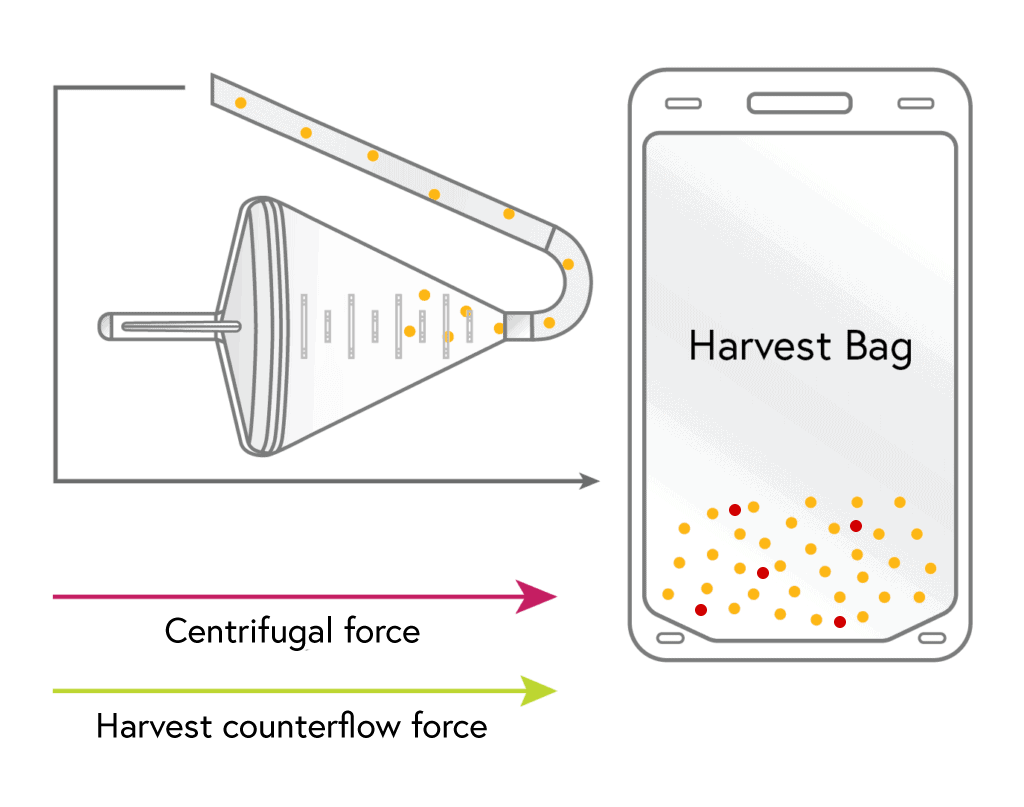
4
HARVEST
The concentrated lymphocytes are then flushed from the chamber into the harvest bag using a small volume of media.
Cell wash and concentrate
Maximizing cell recovery and contaminant removal.
Cells
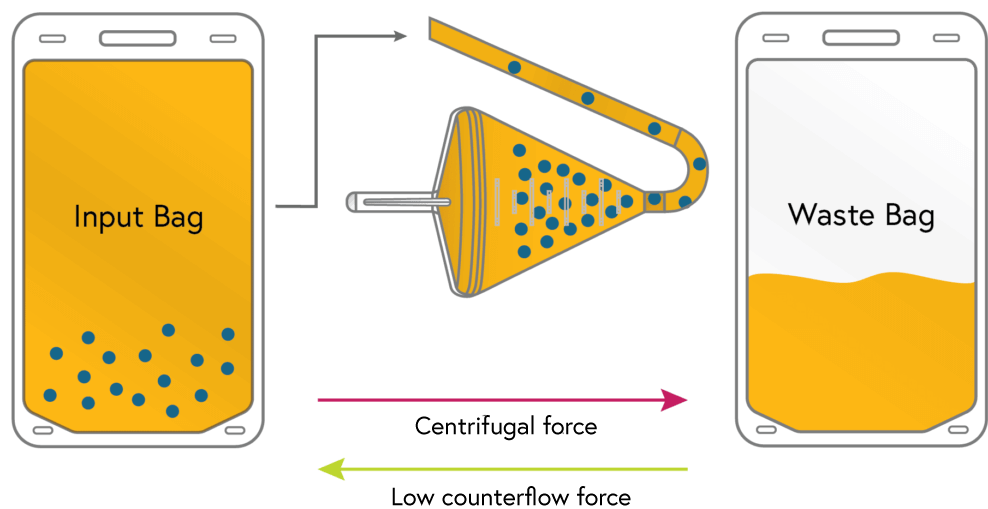
1
Loading
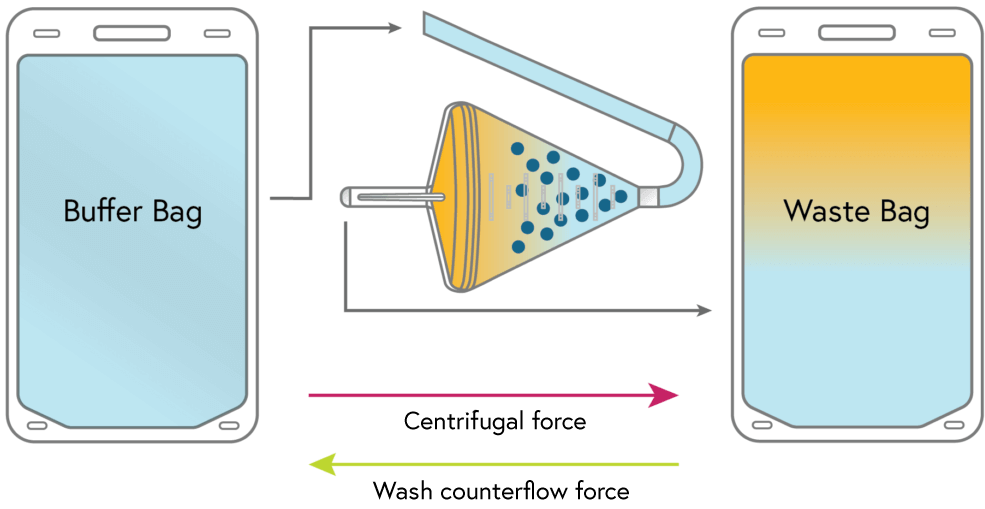
Cells from the input bag are loaded and concentrated in the chamber. Excess fluid from the input bag flows to waste.
2
WASH
With the cells still suspended in the chamber, the remaining starting fluid is completely replaced with a second media. All primary media and any contaminants flow to waste.
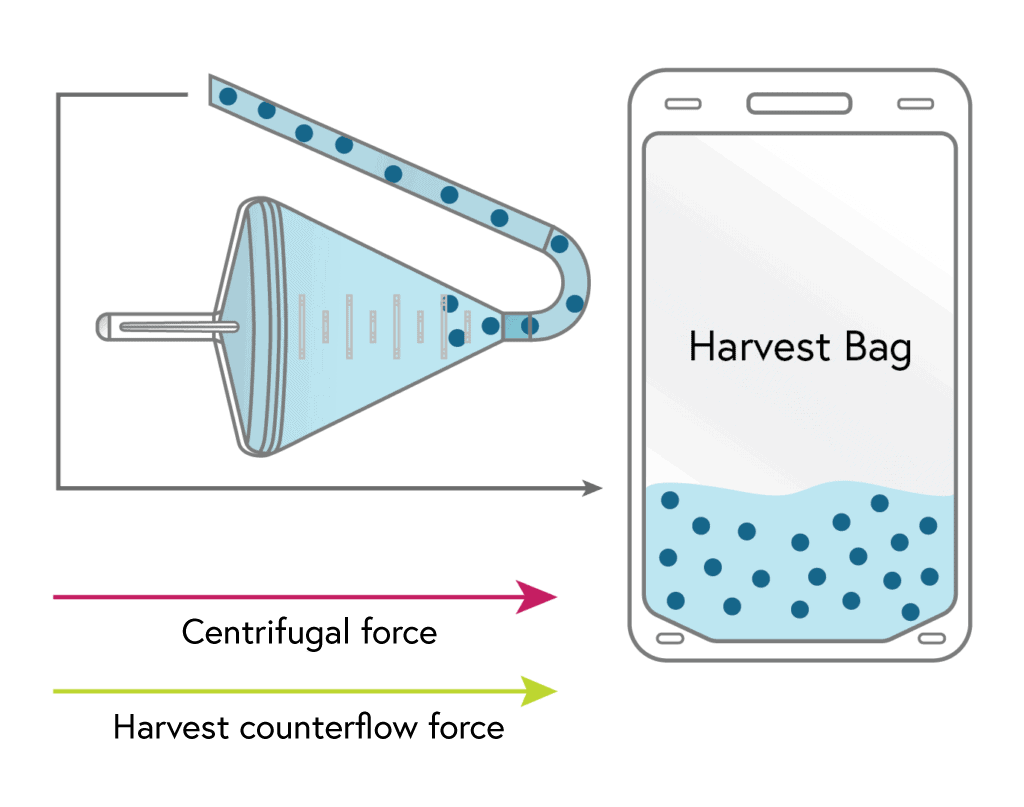
3
HARVEST
Once the media in the chamber is replaced the concentrated cells flow from the chamber to the harvest bag and are suspended in a user selectable volume of secondary media.
*Standard media exchange capability: Korus™ streamlines the manufacturing workflow with the media exchange as well as volume reduction as a standard feature on Korus. Korus can help you achieve the desired cell concentration, volume, and suspension media through a fully automated process.
Use of Korus™ in manufacturing processes
The steps in the process highlighted in green are where the Korus™ would be used.
CAR-T manufacturing process
DC manufacturing process
TIL manufacturing process
IPSC-derived therapy manufacturing process
What is elutriation?
The Korus™ counterflow centrifugation system operates on the principle of elutriation. Elutriation is a process for separating cells based on their size, shape and density using a stream of fluid flowing in the opposite direction to the centrifugal force.
The benefits of elutriation are:
- Separation of target from non-target cells
- Gentle production of a non-pelleting cell bed

Stages in the lymphocyte elutriation process:
1
Loading
Transfer of input cells into chamber(s).
3
Transfer fraction
Transfer of lymphocytes from fraction bag to chamber for concentration.
2
Elutriation
Increased counterflow removes smaller cells (lymphocytes) from chamber(s) to fraction bag.
4
Harvest
Transfer of cells from chamber to harvest bag in a user-defined volume.
Lymphocytes
Monocytes
RBCs
Platelets

The Korus system by Invetech is for research, laboratory or cell therapy manufacturing processes use only. It is not intended as a medical device in therapeutic or diagnostic procedures. Customers are responsible for validating the use of Korus technology within their process or therapy. Consult all instructions, terms and warnings prior to use of the solutions and products. Failure to do so can result in damage or serious injury, including death. Ready-to-run turnkey systems may require minor configuration before sale and use depending on customer needs.