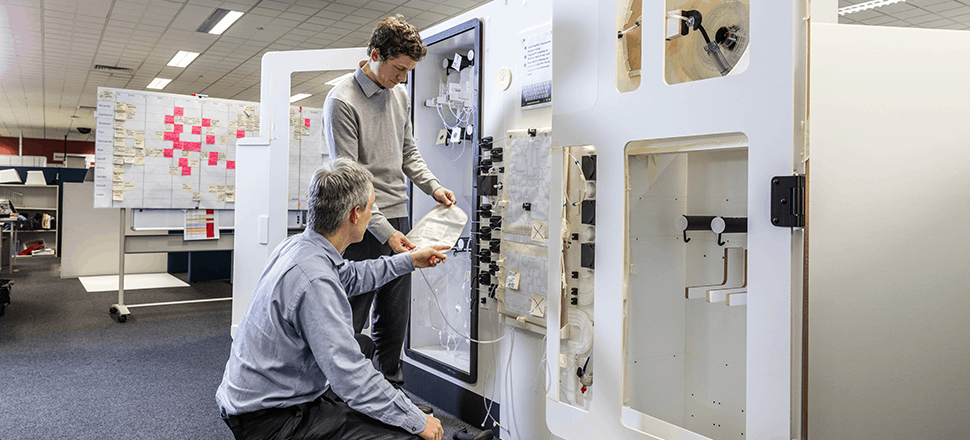
A common challenge in the emerging cell and gene therapy industry is translating manual production methods to clinical and commercial-scale manufacturing. Recently, a clinical-stage biopharmaceutical company was looking to commercialize an autologous Dendritic cell therapy, an immunotherapeutic approach for treating and preventing cancers and autoimmune diseases. The company approached Invetech during Phase II clinical trials to commence development of scaled-out GMP manufacturing solutions.
In briefing Invetech, two key challenges were identified:
- The therapy needed to be produced consistently and repeatedly at a lower cost
- The manual benchtop process was required to be closed in a way that was suited to automation and scale-out yet supported effective biochemistry.
Invetech was engaged to develop a complete manufacturing system that would become the backbone of the client’s entire purpose-built manufacturing facility plans.