Capabilities
Microfluidic Cartridge Design
Microfluidic cartridge design plays a key role in the successful development of a complete IVD system where ease of use, throughput, and cost per test are critical factors. This is particularly true for Point of Care Testing (POCT) applications in which the cartridge also acts as an easy and intuitive user hardware interface.
Design at the start
Invetech matures the microfluidic cartridge design ahead of the instrument by defining key user interactions. This allows maximum flexibility in design and results in a simpler and more effective cartridge concept.
Sometimes IVD instrument development can progress ahead of the consumable. This can be detrimental to the cartridge from a cost and schedule perspective, often requiring a redesign and added complexity, and leading to lower manufacturing yields and higher cost of goods.
This is where Invetech’s approach of balancing all the commercial program success factors as early as possible with the cartridge design can pay the biggest dividends.
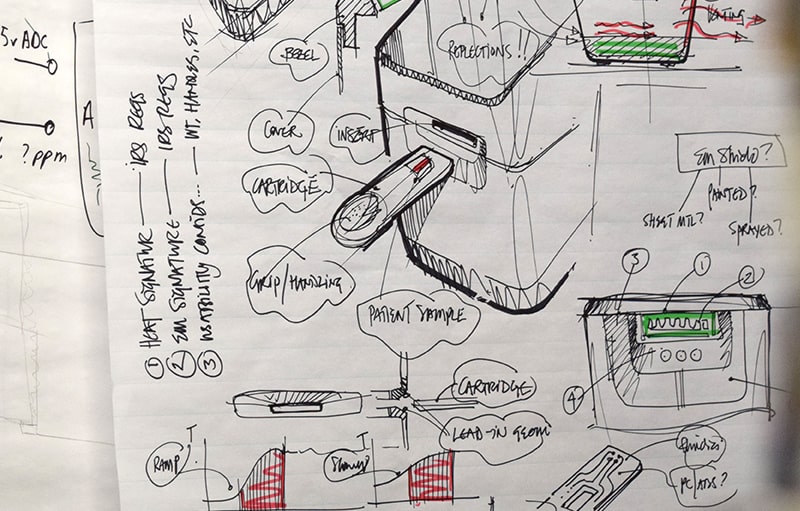
People designing for people
Usability engineering can make the difference between a good and a bad product. Understanding the use environment and what other diagnostic tests users are accustomed to can provide key insights for the design of microfluidic cartridges that are intuitive and easy to use.

Often what seems very straightforward to an engineer can be completely confusing to a typical user. Early thinking about user interactions is critical to creating successful microfluidic cartridge designs. For example, user insights can inform how best to create a sample entry port or easy ways to indicate that the user has adequately filled the cartridge.
Simple things such as providing an obvious and easy-to-grasp handle as well as places for placing a custom label can make the difference between a design that is easy to use and one that is overly complex.
In a POCT system, thinking about ease of use, even for systems not attempting to achieve CLIA Waiver, can help create a better product overall.
Cartridge and product design under one roof
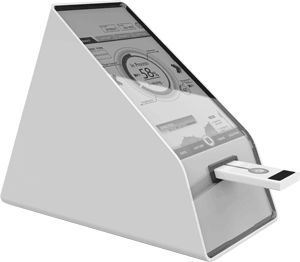 Invetech’s depth of experience in Point of Care product development, spanning instrument, cartridge, and cartridge assembly process and equipment, provides a unique advantage to customers looking to develop a POCT system, such as:
Invetech’s depth of experience in Point of Care product development, spanning instrument, cartridge, and cartridge assembly process and equipment, provides a unique advantage to customers looking to develop a POCT system, such as:
- Identifying risks in the assembly process. We can provide valuable insight into potential risks associated with the cartridge assembly processes based on the design as well as assist with strategies to mitigate those risks.
- Helping make trade-offs on complexity. By looking at the system design holistically, we can help our clients make decisions about where to add complexity and the associated risks and benefits.
- Overcoming technical challenges. We can assist with developing and executing a technical design risk mitigation plan, as well as developing innovative solutions for overcoming specific technical challenges.
- Developing cost estimates and production scale-up strategies. With experience developing automation for high volume consumable production, we can aid in estimating the cartridge cost at various production volumes, as well as planning and executing a production scale-up strategy that balances capital investment and forecast production demand.
- Manufacturing cartridges at low volumes. We have the capability plus assembly and metrology equipment for low-volume cartridge manufacture to support high-volatility development stages.
Invetech, your single point of contact
We have all the requisite skills in-house for both the cartridge and instrument development. This allows the entire project team to have full visibility into the complete development path, key to driving the optimal solution in the fastest time possible, with the least re-iterative efforts.
Critical Considerations in Designing Microfluidic Cartridges
Microfluidic cartridges play a crucial role in healthcare technologies and medical equipment. The design of these cartridges requires careful consideration of various factors to ensure their effectiveness and reliability. This section explores the critical considerations in designing microfluidic cartridges, including design requirements, conceptualization, assembly processes, detailed design, and quality inspection and testing. By addressing these key aspects, healthcare technology organizations and establishments can develop microfluidic cartridges that meet the specific needs of their industries, offering enhanced performance and accurate results.
Design Requirements
Design requirements form the foundation of any microfluidic cartridge development project. It is essential to define and prioritize the key specifications and functionalities that the cartridge should fulfill. Here are some crucial design requirements to consider:
Fluid Compatibility:
Microfluidic cartridges must be compatible with the specific fluids and samples they handle. Analyzing the chemical compatibility, fluid properties, and potential interactions is essential to prevent contamination or damage to the system. Factors such as pH, temperature range, and viscosity should be taken into account during the design process.
In addition, it is important to consider the potential presence of particles or cells within the fluid samples. Cartridge design should account for features such as filters, separation mechanisms, or fluid handling strategies that ensure efficient and reliable processing of the target particles or cells.
Channel Design:
The design of microfluidic channels significantly impacts the functionality and performance of the cartridge. Optimizing fluid flow, minimizing dead volume, and enabling efficient mixing and reaction processes are critical. Parameters such as channel dimensions, geometries, surface treatments, and the inclusion of mixers or valves should be carefully considered to achieve optimal performance.
Furthermore, the choice of channel materials should be based on their compatibility with the fluid, the desired level of transparency for visualization purposes (or level of fluorescence, if applicable), and the fabrication techniques employed. Materials such as glass, silicon, or polydimethylsiloxane (PDMS) offer specific advantages and considerations in terms of biocompatibility, chemical resistance, or ease of fabrication.
Material Selection:
Choosing the right materials is vital to ensure the durability, biocompatibility, and resistance to chemical and thermal stresses of the microfluidic cartridge. It is crucial to evaluate potential issues such as leaching, adsorption, or undesired interactions with the fluids and samples. Materials such as polymers, glass, or silicon may be considered based on the specific requirements of the application.
Polymeric materials, such as poly(methyl methacrylate) (PMMA), cyclic olefin copolymer (COC), or polystyrene (PS), are commonly used due to their cost-effectiveness, ease of fabrication, and compatibility with various fabrication techniques. They offer good optical transparency and can be suitable for disposable or low-cost applications. However, it is important to consider their limitations, such as the potential absorption of small molecules or limited resistance to organic solvents.
In contrast, glass and silicon offer superior chemical resistance and biocompatibility, making them suitable for applications where sample integrity and long-term stability are critical. However, their higher cost, more complex fabrication processes, and limitations in scalability should be taken into account.
Examination:
A thorough examination of the test is vital for the success of cartridge design and implementation. Creating an effective assay involves understanding the properties of the reagents used, such as their thickness, compatibility with other chemicals, and required amount. It’s also important to consider how the reagents will be introduced into the cartridge. Will they come preloaded or in a blister package? The packaging type affects the reagent’s storage, effectiveness, and interaction with other substances. In some cases, it’s preferable to use freeze-dried reagents in microfluidic medical devices to simplify the design and avoid handling liquids.
The characteristics of the reagent determine how the fluid will move in the reservoirs and channels. It’s advisable to make a detailed list of the assay protocol’s features and processes, including the number of reagents, volumes, fluid properties, volatility, compatibility with materials, biocompatibility, and composition (e.g., beads or cells).
Identification:
Identifying the appropriate actuation methods is also crucial. These methods can include positive pressure, vacuum, electrostatic forces, ultrasound, capillary action, or fluidic pumps like diaphragm, piston, film, or peristaltic pumps. The timing and flow rates of actuation are additional important requirements. Since microfluidic processes mimic laboratory workflows on a smaller scale, timing each step accurately at specific volumes is crucial. Some assays have specific timing requirements for each operation, such as sample introduction, mixing, metering, incubation, pre-filtering, separation, sorting, binding, and washing. It’s recommended to list all the unit operations along with the corresponding reagents, volumes, processes, timing, flow rates, and other specific protocol parameters.
By considering these factors in the design requirements, engineers can develop a cartridge concept that efficiently implements the assay protocol while considering manufacturing feasibility and cost.
Conceptualization
The conceptualization phase involves translating the design requirements into a viable microfluidic cartridge concept. This stage includes:
Ideation:
During the ideation phase, brainstorming and exploring different ideas and approaches are crucial. Sketching, 3D modeling, and seeking input from experts in the field can aid in developing innovative and effective designs. Collaboration between engineers, biologists, and clinicians can provide valuable insights and lead to optimized cartridge designs.
Additionally, considering the overall system architecture and user requirements is essential. Understanding the intended application, user workflow, and integration with other components or systems can influence design decisions and ensure a user-centric approach.
System Integration:
Consideration of the overall integration of the microfluidic cartridge within the larger healthcare technology or medical equipment is vital. Understanding the device’s interfaces, connections, and compatibility with external systems is crucial for seamless integration and functionality. Collaborating with system designers and engineers involved in the broader healthcare technology can help ensure smooth integration and optimize the overall performance of the system.
This integration includes factors such as electrical interfaces for sensors or actuators, fluidic connections for sample introduction or waste removal, and communication protocols between the microfluidic cartridge and other system components. Implementing standard interfaces or developing custom adapters and connectors can facilitate interoperability and allow for flexible integration with different platforms or instruments.
Initial design concept:
With specific requirements in mind, we can begin crafting an initial design concept. When using off-the-shelf (OTS) components, it’s crucial to review their specifications and consider their compatibility with cartridge materials and integration feasibility. These components may include pumps, valves, filters, tubes, ports, and interfaces. We should evaluate multiple OTS options, weighing their advantages and disadvantages against building the component from scratch. In most instances, OTS components determine the cartridge’s overall dimensions and performance, so correctly integrating them is vital.
Determining operations:
Another essential aspect of the initial cartridge design is determining the operations that will take place inside it. These operations involve sample ingress and egress, reagent storage, mixing, metering, material filtration, and analyte detection, among others. The design of the sample ingress and egress plays a significant role in user interface design. How the sample is introduced and collected greatly impacts the cartridge’s usability and user experience. Inadequately designed cartridges can be challenging for medical practitioners to handle and may even pose safety risks in certain cases.
Assembly Process
The assembly process is a critical step in the production of microfluidic cartridges. Key considerations include:
Bonding Techniques:
Selecting appropriate bonding techniques to securely join different layers or components together is crucial. Techniques such as thermal bonding, adhesive bonding, or solvent bonding may be employed based on the materials used and the desired bond strength. Ensuring proper bonding is essential to prevent leakage and maintain the structural integrity of the cartridge.
Thermal bonding, also known as thermal fusion bonding, involves applying heat and pressure to bond thermoplastic materials together. It offers strong and reliable bonds, but careful control of temperature, pressure, and alignment is necessary to avoid deformation or delamination.
Adhesive bonding relies on the use of adhesives or tapes to bond different layers or components. It provides flexibility in joining different materials and allows for easy prototyping and reworkability. However, considerations such as curing time, compatibility with the fluids or samples, and long-term stability should be taken into account.
Solvent bonding involves using a solvent or adhesive that can dissolve the surfaces of the materials to be bonded. Upon evaporation of the solvent, the surfaces fuse together, resulting in a strong bond. It is important to select a solvent that is compatible with the materials and does not introduce contaminants into the fluidic channels.
Precision and Alignment:
Precise alignment of channels, ports, and other features is vital to ensure accurate fluid flow and prevent leakage or blockages. Proper alignment can be achieved through advanced manufacturing techniques such as photolithography, laser ablation, or micro-milling. Utilizing high-precision equipment and automated alignment systems can enhance the accuracy and reproducibility of the assembly process.
In addition to alignment, the dimensional accuracy and surface quality of the microfluidic channels and features should be carefully controlled during the manufacturing process. Microfabrication techniques such as lithography, etching, or injection molding can achieve the desired dimensions and surface characteristics, ensuring the smooth flow of fluids and minimizing the risk of fouling or clogging.
Detailed Design
The detailed design phase involves refining the concept into a comprehensive design that can be translated into a physical microfluidic cartridge. Essential aspects to consider include the following:
CAD Design:
Utilizing computer-aided design (CAD) software is essential for creating detailed 2D and 3D designs of the microfluidic cartridge. The CAD design should incorporate the channel geometry, dimensions, fluid inlets and outlets, and any additional features required for the specific application. CAD software allows for accurate visualization, modification, and optimization of the cartridge design before moving to the manufacturing stage.
Advanced CAD tools also offer simulation capabilities, enabling engineers to analyze fluid dynamics, heat transfer, or structural integrity. Simulating the behavior of the cartridge under different operating conditions can aid in optimizing the design, identifying potential issues, and reducing the need for costly and time-consuming iterations during the prototyping stage.
Tolerance Analysis:
Performing tolerance analysis helps determine the allowable manufacturing variations and ensures proper functionality and fit of the cartridge components. Analyzing tolerances for critical dimensions and considering factors such as material shrinkage, manufacturing processes, and assembly variations can aid in avoiding issues such as leaks, blockages, or misalignments.
Using statistical methods or software tools, engineers can evaluate the impact of manufacturing tolerances on the overall performance of the microfluidic chip or system. This analysis helps define the manufacturing requirements, set realistic tolerances, and establish quality control measures to ensure consistent and reliable production.
In the intricate creation of a cartridge, the designer must keep a keen eye on the manufacturing process to be employed. Through careful experimentation and continuous refinement, a set of guidelines can be established to guarantee top-notch manufacturing quality.
To expedite the evolution of cartridge design, a streamlined process allows for swift prototyping. By conducting fluidic tests on a small scale, the performance of various design elements can be evaluated. The initial exploration provides valuable insights for optimizing the cartridge design. This strategy follows a “fail fast” high-speed approach, where detailed design parameters are derived from real-world test data.
Quality Inspection and Testing
To ensure the reliability and performance of microfluidic cartridges, rigorous quality inspection and testing protocols should be implemented:
Leak Testing:
Conducting leak tests is crucial to verify the integrity of the cartridge. This involves subjecting the cartridge to pressure or vacuum conditions to identify any leaks or faulty seals that could compromise the functionality or contaminate the fluids. Techniques such as pressure decay, bubble testing, or dye penetration can be employed to assess the sealing quality of the cartridge.
Automated leak detection systems can provide accurate and efficient testing, ensuring a high level of confidence in the cartridge’s integrity. Performing leak tests at different stages of the manufacturing process, including after bonding or assembly, allows for early detection of issues and timely corrective actions.
Flow Characterization:
Characterizing the fluid flow within the microfluidic system (cartridge) is essential to evaluate its performance and identify potential issues. Techniques such as flow visualization, pressure drop measurements, and particle imaging velocimetry can provide insights into flow behavior, mixing efficiency, and any undesired phenomena such as flow recirculation or clogging. Flow characterization allows for optimizing the channel design and identifying areas for improvement.
Quantitative flow characterization methods, such as computational fluid dynamics (CFD), can simulate and predict fluid behavior within the microfluidic cartridge. This advanced analysis helps optimize the channel geometry, identify potential areas of flow stagnation, and improve the cartridge’s overall performance and amplification of the process.
By addressing these critical aspects, healthcare organizations and medical establishments can develop microfluidic cartridges that meet the specific needs of their industries, offering enhanced performance and accurate results while contributing to the advancement of healthcare technologies and the successful fabrication of industry-leading medical equipment.
What we bring
 Invetech’s capabilities to bring a client’s system to market, complete with cartridge and instrument integration, include:
Invetech’s capabilities to bring a client’s system to market, complete with cartridge and instrument integration, include:
- User experience design and usability
- Industrial design
- Mechanical engineering, including fluidic and microfluidic design and analysis, and reliability planning and durability testing
- Electrical engineering, including MEMS component integration
- Software
- Scientific support, including cell culture and assay development
- Manufacturing, including low-volume assembly line setup and high-volume planning
Get Started
When it comes to cartridge and instrument development, Invetech can help turn your research into reality.