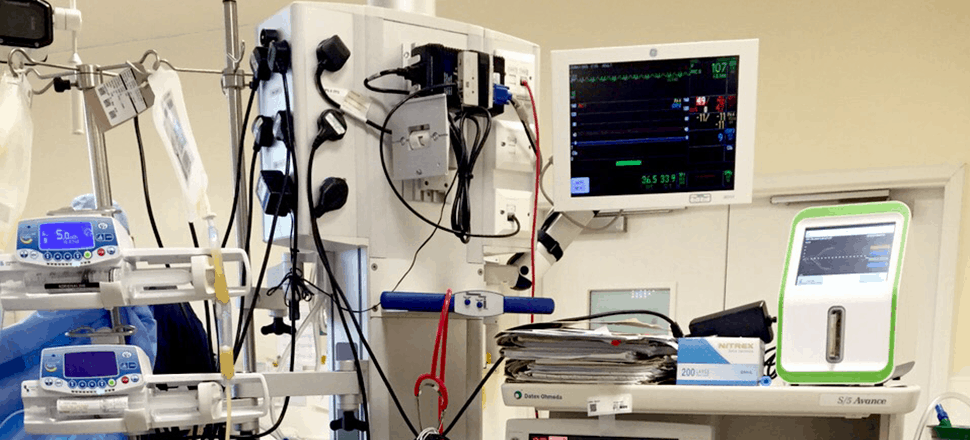
Having real-time awareness of a patient’s blood clotting ability, or hemostasis, in an emergency or surgical setting is crucial for doctors to be able to maintain patient coagulation equilibrium. Oftentimes, hemostasis analysis is completed in a hospital’s central laboratory, separate from the surgical theater. This arrangement wastes valuable time by requiring operating room staff to leave the surgical theater to access these critical test results.
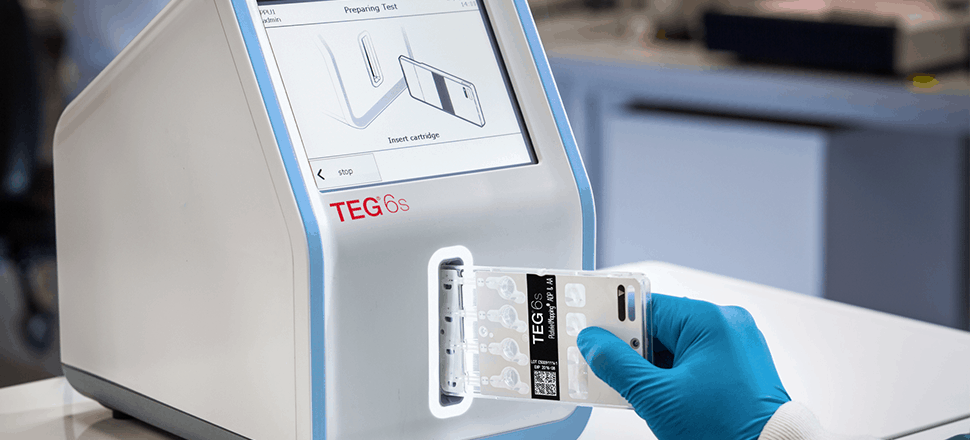
Haemonetics, a healthcare company dedicated to providing innovative blood management solutions, partnered with Invetech to design and manufacture the TEG® 6s Hemostasis Analyzer System. This system is a compact diagnostic instrument that provides clinicians with rapid, comprehensive and accurate identification of a patient’s hemostasis condition in a surgical theater, laboratory or point of care setting.
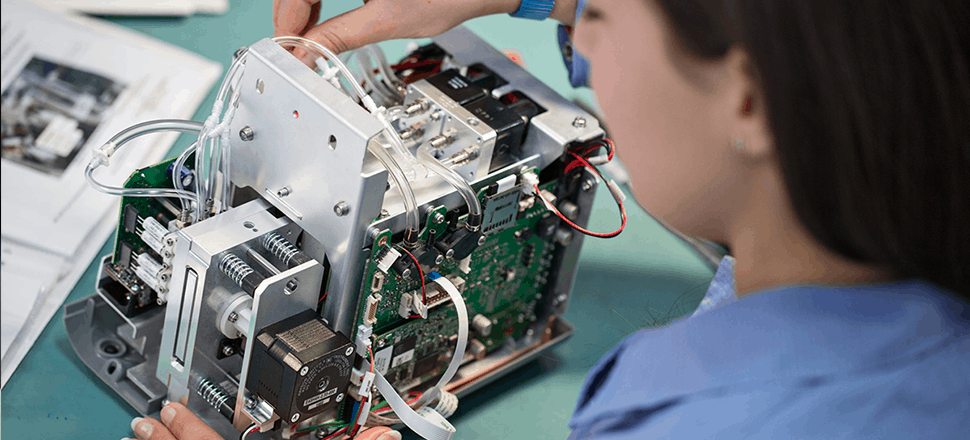
The TEG® 6s Analyzer was introduced to the market in 2014 and has been in full production by our contract manufacturing team since its launch. The instrument went on to win the Good Design Award in 2016 for its compact, minimalist design with all-in-one cartridges, resonance and sensing algorithm, large touchscreen, and user-friendly interface.
Featuring an innovative, all-in-one cartridge, the TEG® 6s system delivers the same quality test results without the complicated test preparation process. The instrument allows clinicians to drive personalized, clinically and economically sound treatment while also allowing them to make decisions with confidence.